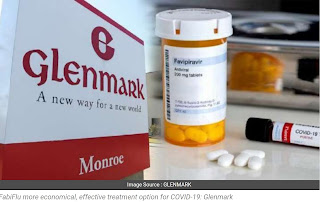
FabiFlu is the first oral favipiravir-approved medication in India for the treatment of COVID-19
Source: IndiaNewsTV.com
More here: https://tinyurl.com/y8g2jyyh
What is FabiFlu?
According to Glenmark, India:
•It is a prescription-based medication, with recommended dose being 1,800 mg twice daily on day one, followed by 800 mg twice daily up to day 14, the drug firm said.
•Available Through Retail & Hospital Channels
•200 Mg Tablet, 34 Tablets in A Strip, ₹103/Tablet ..Strip MRP ₹3500
•The company is producing the active pharmaceutical ingredients (API) for the product at its Ankleshwar plant, while the formulation is being manufactured at its Baddi plant.
•Finally A Good News In Fight Against COVID19. It's Launched Under Brand Name FabiFlu.
•FabiFlu(Favipiravir ) has shown clinical improvement of up to 88 per cent in mild to moderate COVID-19 cases.
Who makes it in India?
Glenmark launches Covid-19 drug at Rs 103 per tablet.
https://en.wikipedia.org/wiki/Glenmark_Pharmaceuticals
Who made the original Favipiravir?
FabiFlu is same as Avigan in Japan. Avigan was developed in 2014 by Fujifilm Toyama Chemical, a unit of Fujifilm Holdings.
According to FujiFilm spokesperson,
“Avigan is a viral RNA polymerase inhibitor, with a new mechanism of action that inhibits viral gene replication within infected cells,”.
Japan is testing Avigan in 43 countries.
More here: https://tinyurl.com/yaek2jxv
No comments:
Post a Comment